Healthcare Professionals
Corvia Atrial Shunt Responders & Patient Selection
Identifying patients with HF with EF≥40% who are responsive to atrial shunt therapy is key to achieving the benefits of the therapy. This process has now been facilitated through the pioneering accomplishments of Corvia’s REDUCE LAP-HF II study.
WHO ARE RESPONDERS?
REDUCE LAP-HF II was the world’s first randomized clinical trial for atrial shunting and defined the optimal patient population for atrial shunt therapy, called “Responders”. Key demographic and hemodynamic characteristics were identified that distinguish “Responders” from “Non-Responders”.

Responders N = 313
Benefitted significantly compared to sham

Non-Responders N = 313
Did significantly worse compared to control
Responder Definition
- No latent pulmonary vascular disease (peak exercise PVR < 1.74 Wood Units)
- No cardiac rhythm device
HOW DO OUTCOMES DIFFER BETWEEN RESPONDERS AND NON-RESPONDERS?
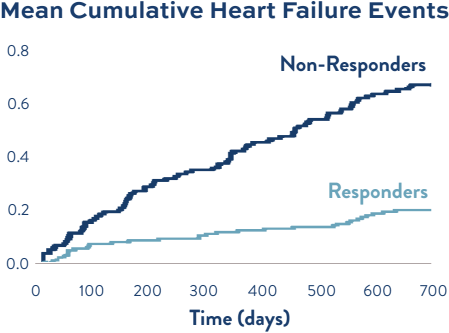
Evidence from REDUCE LAP-HF II demonstrates the importance of appropriate patient selection. Among patients implanted with a Corvia Atrial Shunt, Non-Responders demonstrated a higher rate of HF events in REDUCE LAP-HF II compared to Responders.1
WHAT’S DIFFERENT BETWEEN RESPONDERS AND NON-RESPONDERS?
Key factors about the health of an HF patient with EF≥40% can affect the way left and right heart pressures change in relation to each other when they exercise, which can either favor or disfavor atrial shunt therapy.
Responders
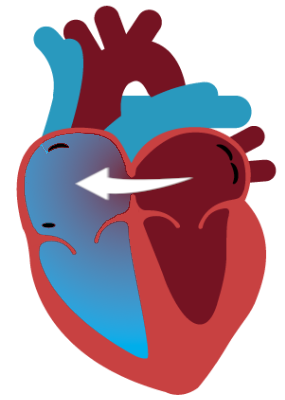
Responders’ relatively healthier pulmonary vasculature, compared to Non-Responders, can accommodate the increase in blood flow to the lungs enabled by atrial shunt therapy. As blood is shunted from the left atrium to the right atrium, the left heart is unloaded, improving systolic function, and the right heart is loaded without worsening its systolic function.
Non-Responders
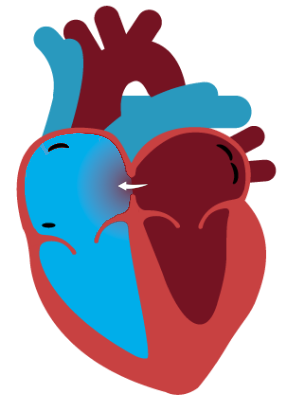
Non-Responders are less able to accommodate the increase in blood shunted from the left atrium due to less compliant pulmonary vasculature. As a result, right heart pressures increase, reducing left-to-right shunting, which limits the benefit of the therapy.
HEAR FROM THE EXPERTS ABOUT THE IMPORTANCE OF PATIENT SELECTION
Sanjiv Shah, MD presents his perspectives on the importance of appropriate patient selection as supported by the results of the RELIEVE-HF trial. Later, Dr. Shah joins a roundtable with Drs. Jim Udelson and C. Michael Gibson for a deeper discussion of the study, including the importance of appropriate patient selection.2
RESPONDER-HF TO VALIDATE ATRIAL SHUNT THERAPY IN HF with EF≥40%
RESPONDER-HF is a confirmatory trial of the Corvia Atrial Shunt in HF patients with an EF≥40% and “Responder” demographics. If you have patients over age 40 with symptomatic HF or unexplained exertional dyspnea, consider sending them for study evaluation.
- Gustafsson, F, Petrie, M, Komtebedde, J, et al. Two-Year Outcomes of an Atrial Shunt Device in HFpEF/HFmrEF: Results from REDUCE LAP-HF II. JACC HF
- https://www.linkedin.com/posts/cmichaelgibson_should-trials-of-interatrial-shunt-technologies-activity-7189337360248045568-ZIhg?utm_source=share&utm_medium=member_desktop