CORVIA® ATRIAL SHUNT SYSTEM
A Novel Treatment for Symptomatic Heart Failure
The only direct treatment for high left atrial pressures (LAP)
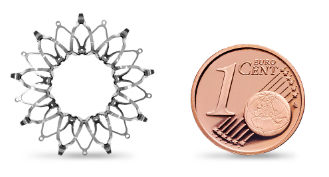
The Corvia Atrial Shunt is about the size of a euro.
The Corvia Atrial Shunt is a novel, minimally invasive cardiac implant for patients suffering from Heart Failure with Preserved Ejection Fraction (HFpEF) or Mildy Reduced Ejection Fraction (HFmrEF). Designed to reduce elevated LAP, the primary contributor to HF symptoms, the Corvia Atrial Shunt offers the most advanced therapeutic option for HF patients with an EF≥40%.
The Corvia Atrial Shunt is the most widely studied interatrial shunt for heart failure. It has been implanted in over 550 patients worldwide, and more than 50 patients have had the shunt for over 5 years.1
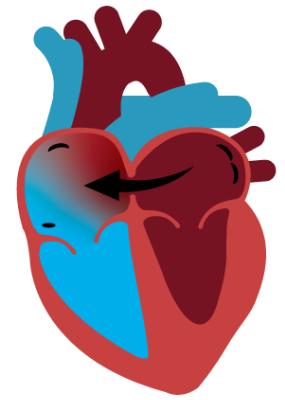
Dynamic Decompression
Atrial shunting safely relieves the high pressures that cause HF symptoms by connecting the two upper chambers of the heart. This connection allows the heart to dynamically decompress by directing blood from the left to right atrium on demand, thereby reducing HF symptoms.
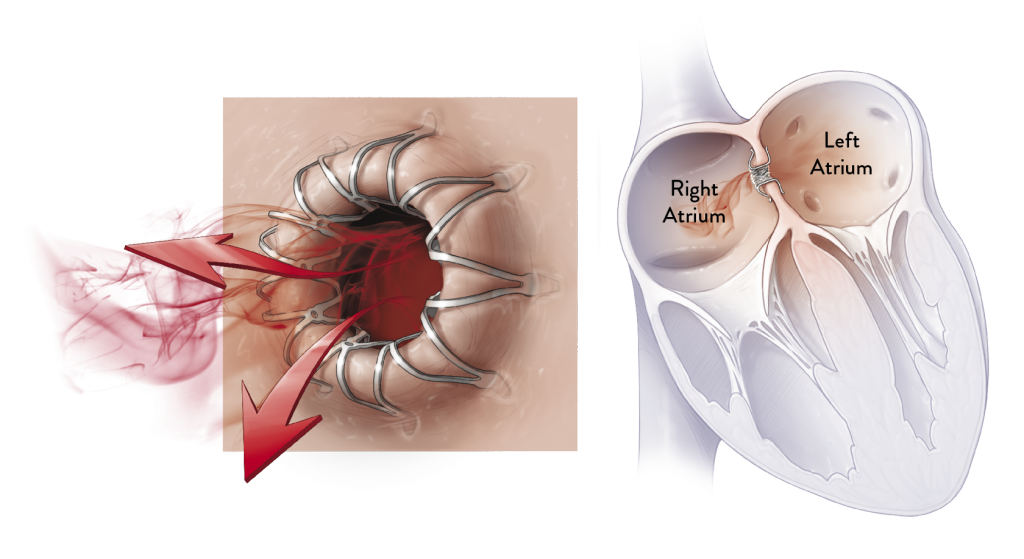
HOW IT WORKS
The Corvia Atrial Shunt is placed by an interventional
cardiologist or electrophysiologist during a one-time
procedure. A catheter is used to place a Corvia Atrial Shunt
as a passage between the left and right atria. This passage
allows blood to flow from the high pressure left atrium to the
lower pressure right atrium, thereby reducing pressure in the
lungs and left side of the heart.
WATCH HOW IT WORKS
By facilitating continuous and dynamic decompression of the left atrium, the Corvia Atrial Shunt has been shown to reduce heart failure hospitalizations and improve heart failure symptoms and quality of life.2,3
71%
After 1 year, 71% of patients had fewer HF symptoms3
75%
After 3 years, 75% of patients reported an improved quality of life4
69%
After 3 years, 69%
of patients had no hospitalizations for heart failure4
IMPORTANT SAFETY INFORMATION
CLICK HERE for Important Safety Information and to access the Instructions for Use.
MAGNETIC RESONANCE (MR) IMAGING INFORMATION
CLICK HERE to access the Instructions for Use

Non-clinical testing demonstrated that the Corvia Atrial Shunt is MR Conditional. A patient with this device can be scanned safely in an MR system immediately after placement under the following conditions:
- Static magnetic field of 1.5 tesla and 3 tesla, only
- Maximum spatial gradient magnetic field of 4,000 gauss/cm (extrapolated) or less
- Maximum MR system reported, whole body averaged specific absorption rate (SAR) of 4 watts/kg for 15 minutes of scanning (i.e., per pulse sequence) in the normal operating mode of operation for the MR system
- Under the scan conditions defined, the Corvia Atrial Shunt is expected to produce a maximum temperature rise of 2.4°C after 15 minutes of continuous scanning (i.e., per pulse sequence).
In non-clinical testing, the image artifact caused by the Corvia Atrial Shunt extends approximately 5 mm from this device when imaged using a gradient echo pulse sequence and a 3 tesla MR system.
- Unpublished data on file at Corvia Medical. Data as of August 1, 2022.
- Feldman T, Mauri L, Kahwash, et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I): A Phase 2, Randomized, Sham-Controlled Trial. Circ. 2018;137(4):364-375.
- Kaye D, Hasenfuß G, Neuzil P, et al. One-Year Outcomes After Transcatheter Insertion of an Interatrial Shunt Device for the Management of Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9(12):e003662.
- Unpublished data from REDUCE LAP-HF (n=64). Data on file at Corvia Medical.
