Healthcare Professionals
A Foundation of Clinical Evidence
The Corvia Atrial Shunt is the most clinically studied atrial shunt for the reduction of Left Atrial Pressure (LAP) in HFpEF/HFmrEF patients. Over 750 patients have been studied to date and multiple studies are ongoing. Through multiple studies around the world, clinical results have consistently established the safety of the Corvia Atrial Shunt System and have demonstrated encouraging efficacy in heart failure patients with an EF≥40% and elevated left atrial pressure.1-2
>550
Corvia Atrial Shunts
implanted globally
>1200
Implanted patient-years of follow-up3
7+
Years post-implant for the longest ongoing Corvia Atrial Shunt patients3
20+
Peer-reviewed publications
on the Corvia Atrial Shunt performance & mechanism of action
Clinical Evidence Summary
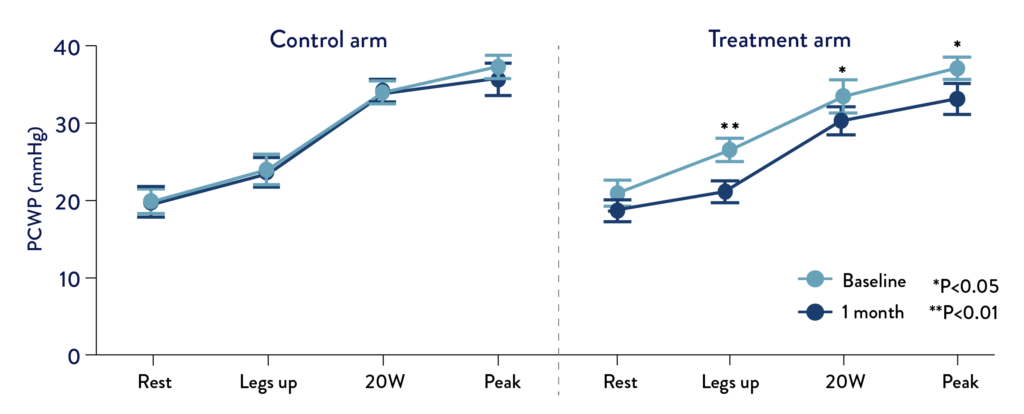
PWCP REDUCTION
Immediate and significant PCWP reduction of 3-5mmHg with clinical improvement sustained over 12 months.4
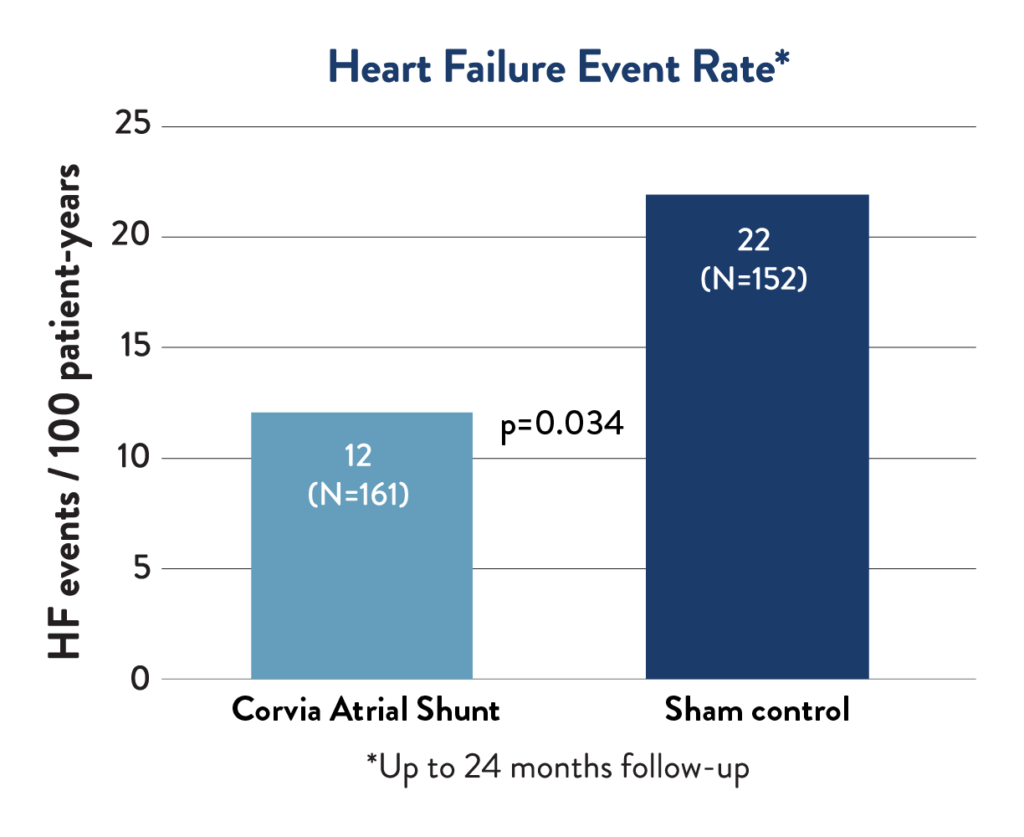
HEART FAILURE EVENTS
Corvia Atrial Shunt therapy led to a 45% reduction in the rate of heart failure events in the REDUCE LAP-HF II Responder subgroup.2
QUALITY OF LIFE
At 12 months, atrial shunt Responder subgroup patients reported a 55% greater improvement over their baseline KCCQ score compared to sham control.2
SAFETY
The Corvia Atrial Shunt demonstrated consistent, durable results in over 550 patients with >1200 patient-years of follow-up.3
>99%
Procedural success
Successful shunt implantation when transseptal puncture is performed for shunt placement
>97%
Freedom from
ischemic stroke5
1.2 events / 100-patient years
>99%
Patency
Flow detected with no echocardiographic evidence of thrombus at one year or beyond6
100%
Implant durability
Freedom from device rejection, embolization (partial or whole), or device removal or closure to reverse therapy
- Kaye DM, Hasenfuß G, Neuzil P, et al. One-Year Outcomes After Transcatheter Insertion of an Interatrial Shunt Device for the Management of Heart Failure With Preserved Ejection Fraction. Circ Heart Fail. 2016;9(12):e003662.
- Borlaug BA, Blair J, Bergmann MW, et al. Latent Pulmonary Vascular Disease May Alter the Response to Therapeutic Atrial Shunt Device in Heart Failure [published correction appears in Circulation. 2022 Jul 26;146(4):e12]. Circulation. 2022;145(21):1592-1604.
- Unpublished data of Jan 1, 2022 compiled from Corvia Clinical Trials and on file at Corvia Medical.
- Feldman T, Mauri L, Kahwash, et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I): A Phase 2, Randomized, Sham-Controlled Trial. Circ. 2018;137(4):364-375
- Analysis through 3 years.
- Core lab unable to analyze flow in a small % of echo studies due to technical quality.