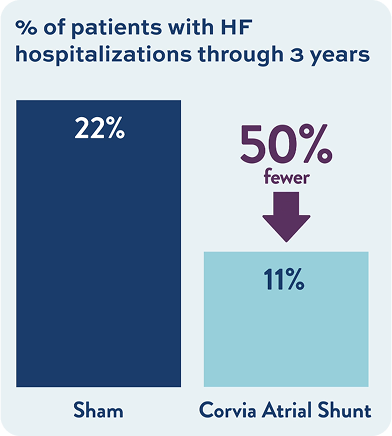
675+ Patients
have received the Corvia Atrial Shunt
18 Countries
in which the Corvia Atrial Shunt has been implanted
100+ Hospitals
with Corvia Atrial Shunt experience
10+ Years
post-implant for the first Corvia Atrial Shunt patients
Seeing is believing
Watch how the Corvia Atrial Shunt is implanted and how it works to reduce heart failure symptoms.
RESPONDER-HF Clinical Study
A clinical trial to confirm the efficacy of the Corvia Atrial Shunt in heart failure patients with EF≥40%
Find a Participating Clinical Trial Center
Find a center that offers the Corvia Atrial Shunt System.