Healthcare Professionals
REDUCE LAP-HF II Trial
LANDMARK TRIAL in HFpEF
REDUCE LAP-HF II is the largest device therapy trial for heart failure patients with preserved ejection fraction (HFpEF), the biggest unmet need in cardiology. This global, landmark, phase III, sham-controlled trial is evaluating the Corvia Atrial Shunt in heart failure patients to reduce HF-related hospitalizations and improve quality of life through a reduction in left atrial pressure (LAP), the primary cause of HF symptoms. Patients will be followed out to 5 years post implant.
The 12-month primary endpoint analyses reinforce the safety and efficacy of the device observed in previous studies and have newly defined treatable patient population for atrial shunt therapy.

Responder Group Highlights
50%
of study patients demonstrated a significant clinical benefit
45%
reduction in heart failure event rate vs. sham control
55%
greater improvement in KCCQ vs. sham control at 12 months
Exercise phenotyping identified as critical for patient selection
Reduce LAP-HF II Trial Design1
PURPOSE: Evaluate the clinical efficacy and safety of the Corvia Atrial Shunt to improve quality of life and reduce HF related symptoms and events in patients with HFpEF or HFmrEF.
Study Population
(N=626 randomized)
- Symptomatic HF
- Ongoing GDMT
- Age ≥40
- LVEF ≥40%
- Preserved RV function
- Elevated exercise PCWP (≥25 mm Hg) with left-to-right gradient (≥5 mm Hg)
- Pulmonary vascular resistance (PVR) <3.5 Wood units at rest or peak exercise
Atrial Shunt Treatment
(N=314)
Sham Control
(N=312)
Trial Outcome
50% of the study population benefited significantly from atrial shunt therapy despite an overall neutral trial2,3
OVERALL POPULATION
Neutral primary outcome when including all patients (win ratio=1)
RESPONDER GROUP (n=313)
Positive outcome in patients with normal exercise PVR (<1.74 WU)4 and no cardiac rhythm device (win ratio5=1.5, p=0.004)
RESPONDER GROUP (n=313)
Positive outcome in patients with normal exercise PVR (<1.74 WU)4 and no cardiac rhythm device (win ratio5=1.5, p=0.004)
OVERALL POPULATION
Neutral primary outcome when including all patients (win ratio=1)
Responder Group Outcomes (N=313)
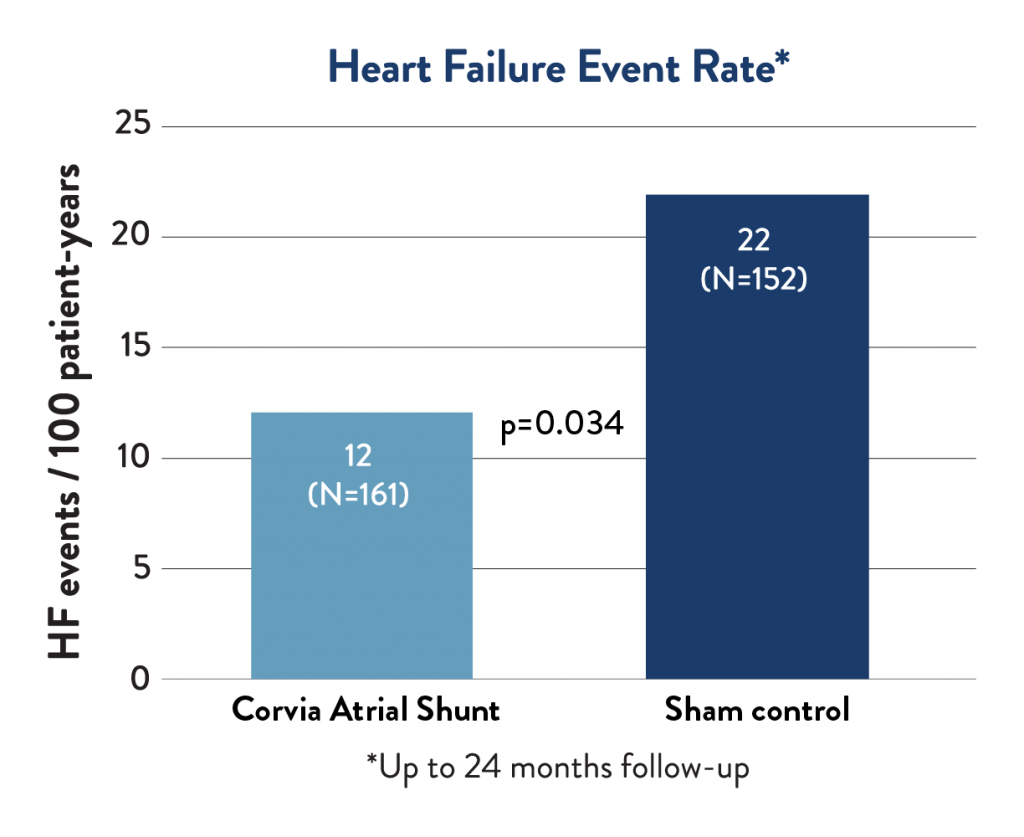
Heart Failure Event Rate
Corvia Atrial Shunt therapy led to a 45% reduction in the rate of heart failure events
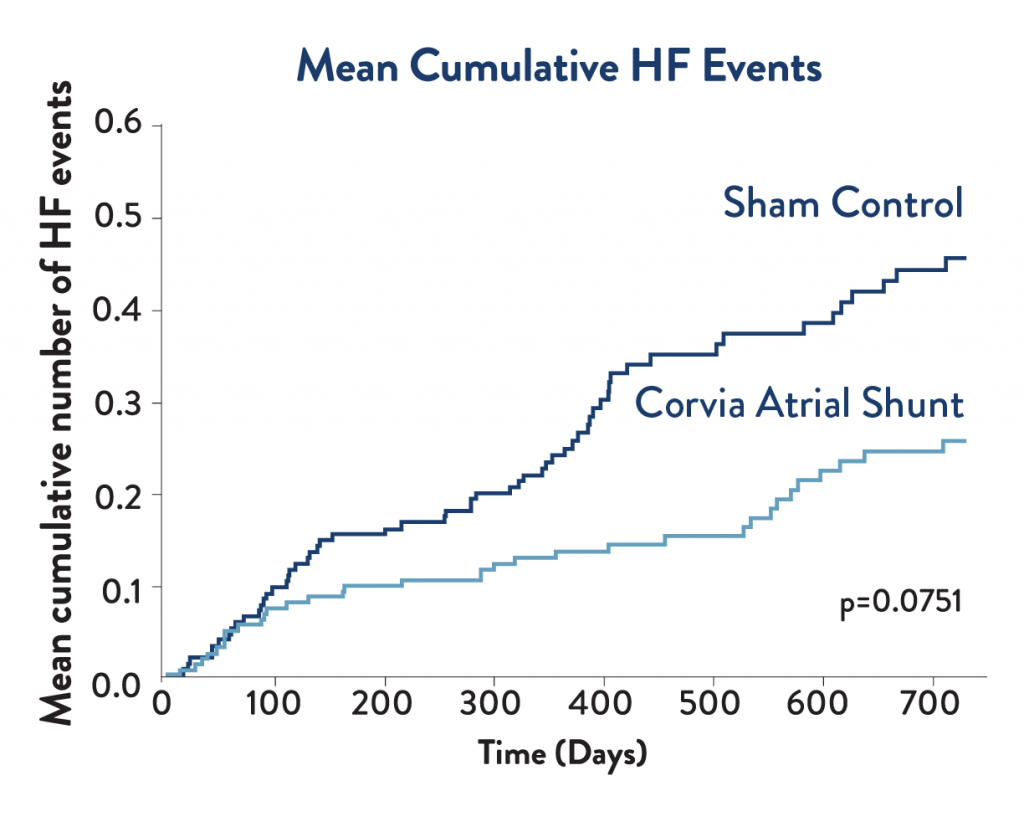
Cumulative Heart Failure Events
Heart failure event curves separated early in the trial and continued to diverge through the 2-year mark
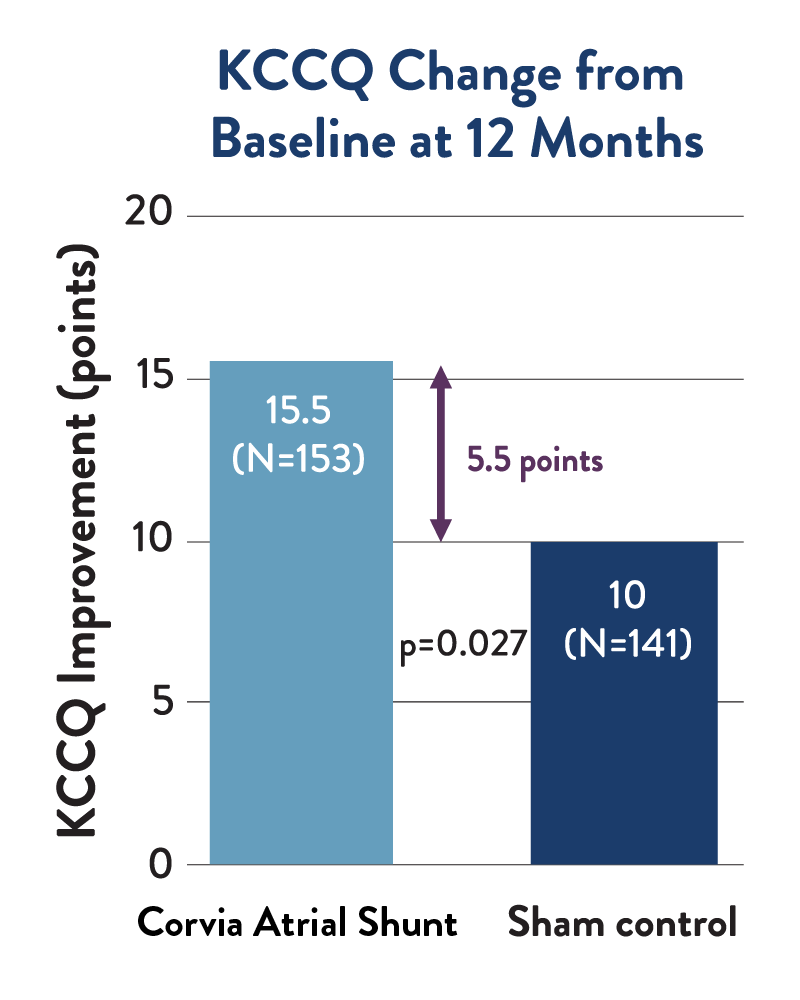
Quality of Life
At 12 months, atrial shunt patients reported a 55% greater improvement over their baseline KCCQ score compared to sham control
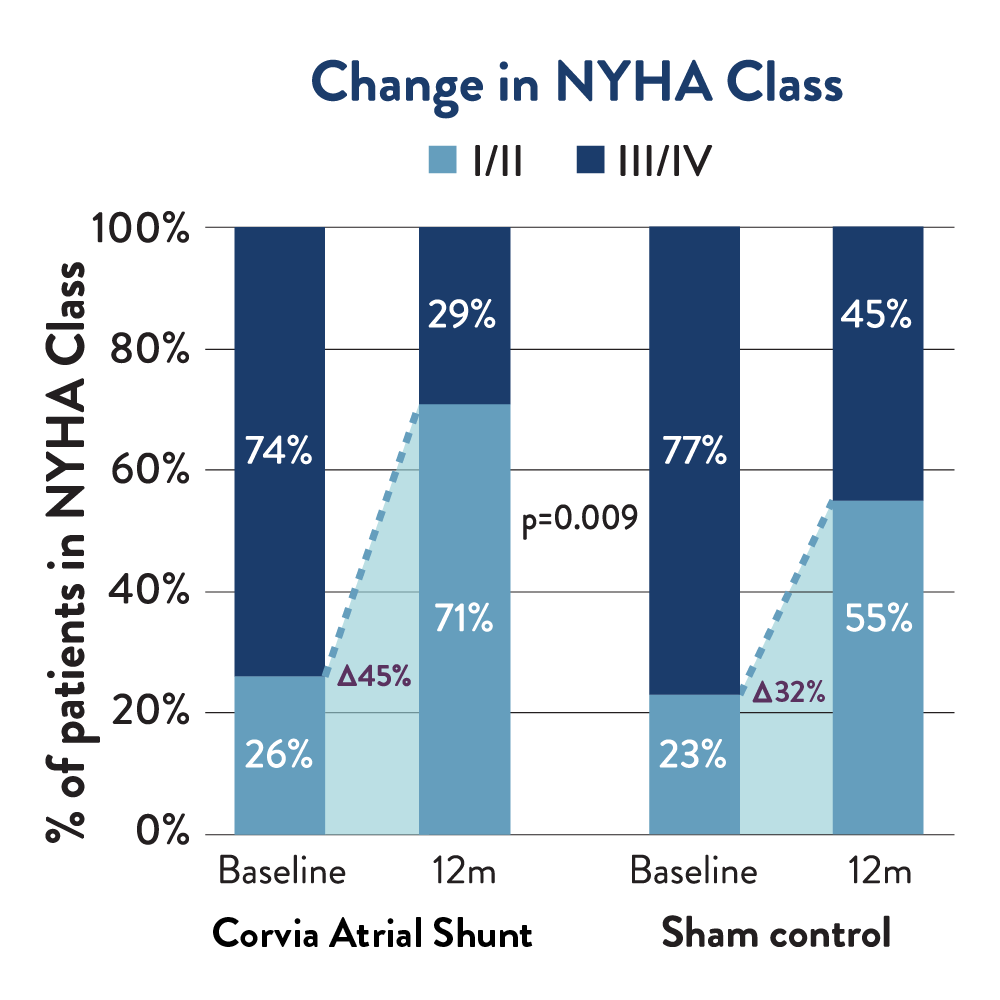
Health Status
Atrial shunt therapy patients were over 40% more likely to improve to NYHA Class I or II compared to sham control
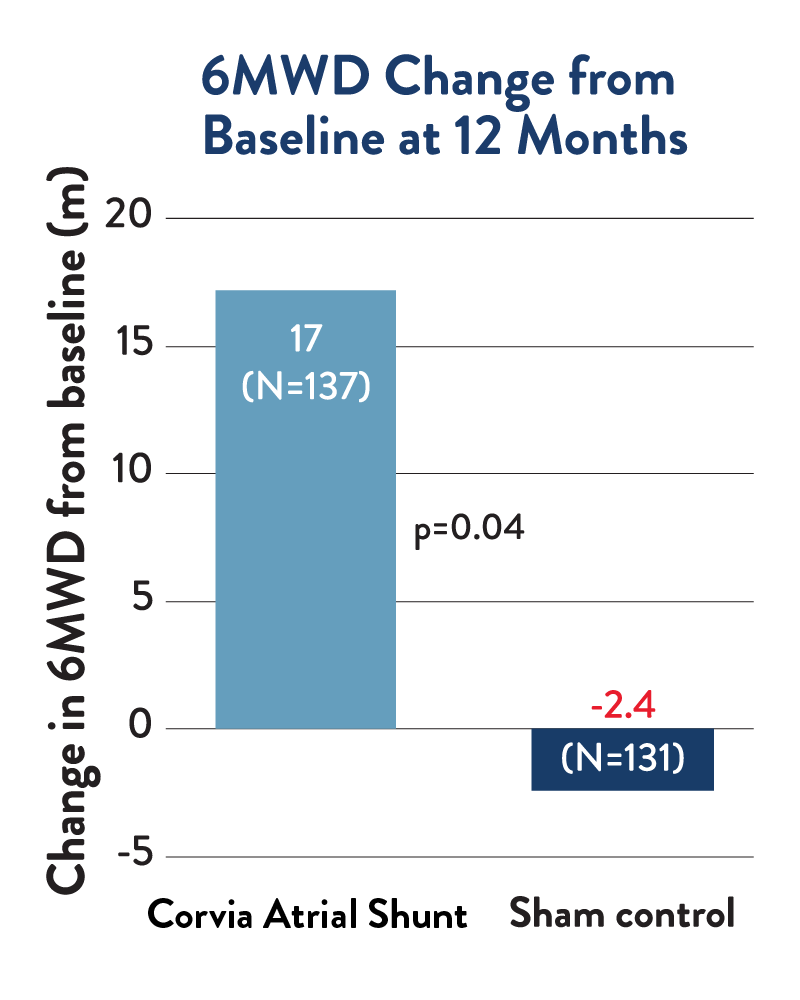
Exercise Capacity
At 12 months, atrial shunt therapy patients significantly improved 6-minute walk distance
Key Safety Outcomes
| Responder population at 1 year | Corvia Atrial Shunt (N=161) | Sham control (N=152) | P-value |
|---|---|---|---|
| Cardiovascular mortality | 1/161 (0.6%) | 0/152 (0.0%) | 0.96 |
| Non-fatal ischemic stroke | 1/161 (0.6%) | 0/152 (0.0%) | 0.96 |
| New or worsening kidney dysfunction | 12/161 (7.5%) | 16/152 (10.5%) | 0.34 |
| Major adverse cardiac events | 3/161 (1.9%) | 0/152 (0.0%) | 0.95 |
| Other thrombo-embolic complications | 0/161 (0.0%) | 0/152 (0.0%) | — |
| ≥30% Decrease in TAPSE | 3/161 (1.9%) | 3/152 (2.0%) | 0.94 |
Circulation Publication
Read the REDUCE LAP-HF II Responder publication in Circulation
Clinical Summary
Download REDUCE LAP HF-II Clinical Summary
Interested in learning more?
Please reach out to your local Corvia field representative or contact us.
- Berry N, Mauri L, Feldman T,. et al. Transcatheter InterAtrial Shunt Device for the treatment of heart failure: Rationale and design of the pivotal randomized trial to REDUCE Elevated Left Atrial Pressure in Patients with Heart Failure II (REDUCE LAP-HF II). Am Heart J, 2020; 226:222-31.
- Shah SJ, Borlaug BA, Chung ES, et al. Atrial shunt device for heart failure with preserved and mildly reduced ejection fraction (REDUCE LAP-HF II): a randomised, multicentre, blinded, sham-controlled trial. Lancet. 2022;399(10330):1130-1140.
- Borlaug, BA, Blair, J, Bergmann, MW et al. Latent Pulmonary Vascular Disease May Alter the Response to Therapeutic Atrial Shunt Device in Heart Failure. Circulation. 2022;10.1161. (in press)
- Upper tertile, which roughly corresponds to peak exercise in a healthy adult >55 years (≤1.8WU).
- In win ratio calculation, all patients are compared with each other in pairwise manner on values of the components in a hierarchical manner (1 = neutral, >1 treatment better, <1 sham better).